Pharmacodynamics is the study of how drugs have effects on the body. The most common mechanism is by the interaction of the drug with tissue receptors located either in cell membranes or in the intracellular fluid. The extent of receptor activation, and the subsequent biological response, is related to the concentration of the activating drug (the ‘agonist’). This relationship is described by the dose–response curve, which plots the drug dose (or concentration) against its effect. This important pharmacodynamic relationship can be influenced by patient factors (e.g. age, disease) and by the presence of other drugs that compete for binding at the same receptor (e.g. receptor ‘antagonists’). Some drugs acting at the same receptor (or tissue) differ in the magnitude of the biological responses that they can achieve (i.e. their ‘efficacy’) and the amount of the drug required to achieve a response (i.e. their ‘potency’). Drug receptors can be classified on the basis of their selective response to different drugs. Constant exposure of receptors or body systems to drugs sometimes leads to a reduced response (i.e. ‘desensitization’).
CPT-01-02-01 What are the mechanisms of drug action?
CPT-01-02-07 Selectivity
CPT-01-02-02 What are receptors?
CPT-01-02-03 What are non-receptor targets?
CPT-01-02-05 What are agonists and antagonists?
CPT-01-02-04 Dose-response-relationships
CPT-01-02-06 Efficacy-potency
CPT-01-02-09 Therapeutic index
Introduction to pharmacodynamics
Clinical pharmacodynamics is the study of:
- the biochemical and physiological effects of drugs on the body
- the mechanisms of drug action in the body
- the relationship between drug concentration and drug effect.
Clinical pharmacodynamics can be simply described as the study of ‘what a drug does to the body’. Basic pharmacodynamic studies involve exposing cells or tissues to constant concentrations of a drug and observing its effect. For clinicians who prescribe drugs, the situation is more complex, because drug exposure depends on how effectively drug molecules are taken into the body and reach their site of action (absorption, distribution) after discrete doses and how quickly they are removed from the body (metabolism, excretion). These processes are collectively known as pharmacokinetics (the study of ‘what the body does to a drug’).
The pathophysiology underlying the progression of most diseases involves disordered structure and function of cells and tissues, which are composed of complex molecules and biochemical processes. Drugs act to restore normal function by acting on ‘target molecules’ in the affected tissue or organ. Binding exerts a biological effect, either by initiating new events or by blocking the actions of endogenous substances (e.g. neurotransmitters, hormones). Resulting effects include changing the ion content of cells, promoting secretion of hormones, reducing the electrical signalling by excitable cells, reducing contractile activity, stimulating the synthesis of new proteins, and many others. Most of these responses result from interactions between a drugs and endogenous ‘receptors’.
RESOURCES
This link will download a pdf that introduces ligand-target interactions (at receptors, ion channels and enzymes), basic receptor theory (agonist and antagonist interactions) and the concept of affinity. It also provides an elementary learning exercise. This may be appropriate as an introduction to the topic followed by more in-depth discussion or examples in a classroom setting for instance. This article was written by Deborah Robertson, senior lecturer and programme leader non-medical prescribing, University of Chester (email: d.robertson@chester.ac.uk).
This is a pdf of a slide set covering many topics in pharmacodynamics including receptor theory, relation between drug concentration and response, potency and efficacy, antagonists – competitive and noncompetitive, partial and full agonists, spare receptors, receptor-mediated signaling systems, receptor desensitization, quantal dose-effect curves, and therapeutic index. Self-assessment questions are interspersed throughout. Equations related to the law of mass action are used making this resource more appropriate for beginning graduate students in pharmacology but clinical correlations are included as well.
Desensitization
Desensitization refers to the common situation where the biological response to a drug diminishes when it is given continuously or repeatedly. It may be possible to restore the response by increasing the dose (or concentration) of the drug but, in some cases, the tissues may become completely refractory to its effect. The term tachyphylaxis is used to describe desensitization that occurs very rapidly, sometimes with the initial dose. The term tolerance is conventionally used to describe a more gradual loss of response to a drug that occurs over days or weeks. There is no clear distinction but the different scales imply that different mechanisms may be involved. Rapid development implies the depletion of chemicals that may be necessary for the pharmacological actions of the drug (e.g. a stored neurotransmitter released from a nerve terminal) or receptor phosphorylation. Slower development implies changes in receptor numbers or the development of counter-regulatory physiological changes that offset the actions of the drug (e.g. accumulation of salt and water in response to vasodilator therapy) or reduction of target receptor numbers.
The term refractoriness is often used to describe a state where there is lack of responsiveness to a drug. Drug resistance is a term normally reserved to describe the loss of effectiveness of an antimicrobial or cancer chemotherapy drug. In addition to these pharmacodynamic causes of diminished effectiveness, reduced response may be the consequence of a reduction in the plasma and tissue drug concentrations that result from the same drug dose, because of alterations in the way the drug is handled (its ‘pharmacokinetics’).
Where desensitization to a drug arises because of established chemical, hormonal and physiological changes that offset the actions of the drug, discontinuation may mean that these changes themselves cause ‘rebound’ withdrawal effects (e.g. nitrates, opioids, benzodiazepines).
Dose-response curves
If the drug dose is plotted on a base 10 logarithmic scale, this produces a sigmoidal dose-response curve. This representation is more useful because it expands the dose scale in the region where drug response is changing rapidly and compresses the scale at higher doses where large changes have little effect on response. Note that, in reality, it is ligand concentration (and resulting receptor occupation) that affects response – the term ‘dose-response curve’ assumes that the drug dose and ligand concentration are closely linked.
Drug dose-response involves the principles of pharmacokinetics and pharmacodynamics and can be used to determine the required dose and frequency to achieve the desired response, for example the desired drug effect. Multiple factors can cause variation in dose-response curves: population differences, patient-related factors and measurement methodologies for example, but repeated measurements carried out under identical conditions can help establish the pharmacologic profile of the drug being evaluated. The dose-response relationship is important because the concentration of a drug at its site of action controls its effect, with caveats as mentioned above..
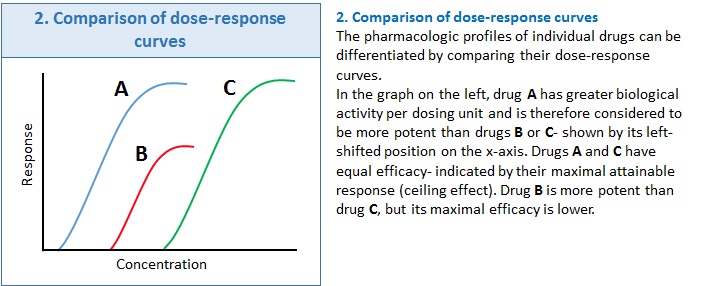
Box 2 shows how drug profiles can be compared based on their plotted dose-response curves.
Mechanisms of drug action
Normal physiology depends on the structure and function of cells and tissues, which are composed of complex molecules and biochemical processes. The pathophysiology underlying the progression of most diseases involves disordered activity of one or more of these cells. Drugs are exogenous molecules that are intended to restore the activity of the endogenous systems to normal by increasing or decreasing the activity of key regulatory pathways. They do this by exerting effects on cells and tissues, either by initiating new events or blocking the actions of endogenous substances, to exert a biological effect. These biological responses include changing the ion content of cells, promoting secretion of hormones, reducing the electrical signalling by excitable cells, reducing contractile activity, stimulating the synthesis of new proteins, and many others. Most of these responses result from interactions between a drugs and endogenous receptors.
Receptors are glycoproteins situated in the cell membrane (or at an intracellular site) that specifically recognise and bind ligands – smaller molecules capable of ‘ligating’ themselves to the receptor protein. The ligands of interest in pharmacology are exogenous compounds (i.e. drugs), receptors in human tissues have evolved to bind endogenous ligands such as neurotransmitters, hormones, and growth factors. When ligands bind to receptors they initiate a conformational change in the receptor protein that eventually transmits a biochemical signal into the cell (signal transduction), sometimes involving a secondary messenger, which is ultimately translated into a biological response (e.g. muscle contraction, hormone secretion). Drug-receptor complex formation is reversible with the biological response being proportional to the fraction of receptors bound.

This is a sample chapter from the textbook, Pharmacology for Nurse Anesthesiology (Ouellette and Joyce, 2011, ISBN-10: 0763786071). In 10 pages, it provides a relatively comprehensive discussion of pharmacodynamics. The last section discusses briefly Mechanisms of General Anesthesia.